

ICH stability test chamber aims to assess the shelf life of pharmaceutical products according to ICH Guidelines. It involves testing the drug product under various environmental conditions, such as temperature, humidity and light, to assess drug reaction over time. The results of the testing are used to determine the warranty period of the drug product.
Model: TG-800GSP
Capacity: 800L
Shelf: 4 pcs
Color: Off white
Interior dimension: 800×590×1650 mm
Exterior dimension: 1360×890×2000 mm
Climatest Symor® drug stability test chamber follows ICH Guidelines, it is a type of climatic chamber used to test the stability of pharmaceutical products. It is designed to simulate the environmental conditions that drugs may be exposed to during storage and transport. The ICH Guidelines provide guidance on the design and operation of the drug stability test chamber, as well as the testing protocols that should be used.
Specification
|
Model |
TG-150GSP |
TG-250GSP |
TG-500GSP |
TG-800GSP |
TG-1000GSP |
|
Interior Dimension ( W*D*H) |
550×405×670 |
600×500×830 |
670×725×1020 |
800×590×1650 |
1050×590×1650 |
|
Exterior Dimension ( W*D*H) |
690×805×1530 |
740×890×1680 |
850×1100×1930 |
1360×890×2000 |
1610×890×2000 |
|
Capacity |
150L |
250L |
500L |
800L |
1000L |
|
Temperature Range |
Without light 0~65°C, With light 15~50°C |
||||
|
Temperature Fluctuation: ±0.5°C; Temperature Uniformity: ±2.0°C |
|||||
|
Humidity Range |
35% ~ 95% R.H |
||||
|
Humidity Deviation |
±3.0% R.H |
||||
|
Lighting |
0~6000LX adjustable ≤±500LX (Unlimited adjustment of light intensity) |
||||
|
Temperature Control |
Balanced temperature adjustment method |
||||
|
Humidity Control |
Balanced humidity adjustment method |
||||
|
Refrigeration |
Two sets of independent original imported hermetic compressors switchover automatically (LHH-80SD: one set) |
||||
|
Interior Material |
Anti-corrosion SUS#304 brushed stainless steel |
||||
|
Exterior Material |
Cold rolled steel plate with electrostatic powder spraying |
||||
|
Insulation |
Superfine fiberglass wool / polyurethane |
||||
|
Controller |
Programmable LCD controller |
||||
|
Sensor |
PT100 platinum resistance / Capacitive humidity sensor |
||||
|
Shelves |
3PCS |
3PCS |
4PCS |
||
|
Power Consumption |
2100W |
2300W |
3750W |
7150W |
7150W |
|
Power Supply |
220V/50HZ |
380V/50HZ |
|||
|
Insert MIni Printer |
1 set |
||||
|
Protection Devices |
Compressor overheat protection, fan overheat protection, over-temperature protection, compressor overpressure protection, overload protection, water shortage protection. |
||||
|
Working Condition |
+5~30℃ |
||||
Safety protection:
·Independent temperature limiter: An independent shutdown and alarm for thermal protection purpose during the test.
·Refrigeration system: Over-heat, over-current and over-pressure protection of compressor.
·Test chamber: Over-temperature protection, overheat of fan and motor, phase failure/reverse, timing of the entire equipment.
·Others: Leakage and outage protection, overload fusing protection, audio signal alarm, power leakage protection, and overload Protection.
Temperature and humidity curve:
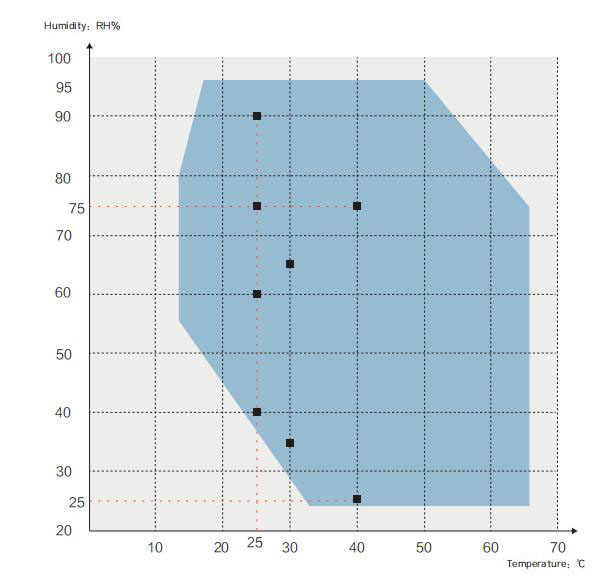
■Pharmacopoeia Drug Stability Guidelines of Raw Drugs and preparations and the
temperature and humidity test conditions required in the ICH guidelines:
The ambient temperature for the following tests shall be between 15~25℃
√Accelerated test: 40℃±2℃ / 75%±5%RH, or 30℃±2℃ / 65%±5%RH
√High humidity test: 25℃ / 90%±5%RH, or 25℃ / 75%±5%RH
√Long-term test: 25℃±2℃ / 60%±5%RH, or 30℃±2℃ / 65%±5%RH
√For accelerated testing of drug preparations packaged in semi-permeable
containers, such as infusion bags prepared by LDB, plastic ampoules, and ocular
preparation containers etc,tests shall be performed at temperature 40℃±2℃/25%±5%RH
√For long-term testing of pharmaceutical preparations packaged in semi
permeable containers, it should be at a temperature of 25℃±2℃/40%±5%RH or 30℃±2℃/35%±5%RH
Feature
What are the main features of a ICH Stability Test Chamber?
The following may help you better understand this chamber:
1. Temperature control: ICH Stability Test Chamber can maintain precise temperature control, the temperature range can be as low as -20°C to as high as 70°C.
2. Humidity control: The humidity level inside the stability pharmaceutical chambers can be set to simulate different humidity. This is particularly important for moisture sensitive drugs, such as certain types of solid dosage forms and biologics.
•Programmable touch screen controller
. 100 programs, 1000 segments 999 steps, 99 hours 59 minutes for each segment.
. P.I.D automatic calculation function.
. RS485 communication interface / a built-in printer available, for data storage and playback of history curve.
. Data recording and fault diagnosis display, once a fault occurs, the cause of fault will be dynamically displayed on the controller.
3. Lighting control: Some drugs are light sensitive, and may degrade if exposed to certain wavelengths of light. Therefore, Climatest Symor® drug stability test chamber has lighting controls, like UV light, to determine the effect of light on the drug product.
4. Air circulation: ICH Stability Test Chamber has air circulation systems to maintain consistent and uniform temperature and humidity throughout the chamber.
5. Data logging and monitoring: ICH Stability Test Chamber is equipped with sensors and data logging systems that monitor and record temperature, humidity, and other environmental parameters, which can be used to generate reports and validate the stability of the product.
Overall, stability testing chamber is designed to ensure that the drugs are stored and tested under controlled environmental conditions that simulate real-world conditions, and to provide accurate and reliable stability data for regulatory approval.
Testing area:
The testing area of a pharmeceutical stability testing chamber is made of brushed stainless steel SUS304, and is designed to simulate constant temperature, humidity, or lighting conditions. The chamber is equipped with high-precision temperature & humidity sensors to monitor and maintain these climatic conditions.
There are racks or shelves to hold the drug samples, these shelves are height adjustable, and the samples are usually placed in tightly sealed glass vials or containers to prevent contamination.

Benifits offered by ICH Stability Test Chamber
ICH Stability Test Chamber offers many benefits to pharmaceutical manufacturers, including:
. Ensuring product quality: Stability chamber in pharma helps pharmaceutical companies test and study their products quality, which is critical for ensuring that they remain safe and effective throughout their shelf-life.
. Meeting regulatory requirements: Stability testing is a critical part of the regulatory approval process for pharmaceutical products, and stability chamber in pharma is essential to meet regulatory requirements.
. Enhancing manufacturing efficiency: Stability testing can also provide valuable data on the shelf-life of new product formulations, which can inform product development and optimization efforts.
. Reducing product waste: Stability testing can help identify products that are at risk of degrading or becoming unstable, which can help manufacturers save manufacturing cost.
In summary, ICH Stability Test Chamber helps to ensure product quality, comply with regulatory requirements, cost-effective testing, improve product development, and increase productivity.
The role of a ICH Stability Test Chamber
ICH Stability Test Chamber is designed to meet strict regulatory requirements and industry standards, such as those set by the International Conference on Harmonisation (ICH Guideline). The chambers can be used for a variety of purposes, including:
*Long-term storage stability testing: This type of testing is used to determine the stability of a drug over an extended period of time, typically several years.
*Accelerated stability testing: This type of testing is used to evaluate the stability of a drug under harsh conditions, such as high temperatures and humidity, in a short time.
* Shelf life testing: ICH Stability Test Chamber is used to determine the shelf life of a drug, which is the amount of time a product can be stored under specific conditions without losing its potency, efficacy, or quality.
Based on the results of stability testing, the manufacturer can determine the shelf life of the product and make necessary adjustments to the formulation or packaging, to ensure the product remains stable over time. This data is critical to regulatory agencies, who use it to determine the appropriate storage and handling requirements for the drugs.

Accelerated testing in a ICH Stability Test Chamber
Accelerated testing is carried out under regular conditions, and its purpose is to speed up the chemical or physical changes of the drug for drug review, packaging, transportation and delivery. Below is an example to show the accelerating test procedures:
Applicable products: Raw materials and pharmaceutical preparations
Batches: 3 batches, market packaging
Storage condition: 40℃±2℃; 75%±5%
Storage time: 6 month
Assessment: Take out samples from the 1st, 2nd, and 3rd batches after 6 months, inspect them according to the established quality standards, if they do not meet the standard, then test @30°C±2°C, 65%+5% for 6 month.
Temperature sensitive drugs are expected to be stored in the refrigerator (4~8°C). The accelerated test can be carried out @25°C±2°C; 60%±10%, 6 month.
Certificates of Climatest Symor® ICH Stability Test Chamber
Certificates are official documents issued by the manufacturers or accredited by the third-party organizations, it verifies the performance and compliance of the chamber with relevant regulations and standards. Climatest Symor® is ISO9001:2015 certified, all stability test chambers are CE approved.

On-site installation pictures
Installing a ICH Stability Test Chamber requires careful planning and attention to detail to ensure that it is properly installed
